National Institute for Health and Care Excellence (“NICE”) guidelines announce guidance supporting the use of Neovasc Reducer™ System therapeutic intervention in the treatment of refractory angina
London, November 26, 2021
Today, the National Institute for Health and Care Excellence (“NICE”) has recommended the use of Neovasc Reducer™ System in suitable patients suffering from refractory angina. This is a breakthrough for refractory angina patients, many of whom suffer debilitating chest pain as alternative treatment options have failed or are not a viable option.
NICE has restricted its’ recommendations to the psychological impact of patient’s pain. This recommendation is a positive step forward in improving patient outcomes, as NICE has previously not supported any therapeutic interventions in the treatment of refractory angina. The placement of the Reducer is a transvenous procedure performed in the Cardiac Catherisation lab which takes approximately 20 minutes with limited post procedure side-effects.
In accordance with NICE guidelines, “Coronary sinus narrowing device implantation is indicated for people in whom other treatment options (medical or surgical) have failed or are not possible (refractory angina). The aim is to reduce symptoms and to improve quality of life.”
Dr. Jonathan Hill, MD, Consultant Interventional Cardiologist at Royal Brompton Hospital, London, commented, “The NICE committee that evaluated the coronary sinus narrowing device extensively reviewed the available clinical data regarding the Reducer device. Today’s guidance document is good news for patients in the United Kingdom suffering from refractory angina that haven’t had treatment options supported by NICE guidance.”
Nathan Pettitt, Group General Manager at Healthcare 21 commented “Today is a fantastic day for our cardiovascular team at Healthcare 21, Neovasc and most importantly patients across the UK. Our team has worked tirelessly to launch the innovative Neovasc Reducer™ system in the treatment of refractory angina. Our partnership with Neovasc Inc. is an important one and this NICE recommendation will ultimately result in continued application of the Neovasc Reducer™ and ultimately improved outcomes for patients across the UK.”
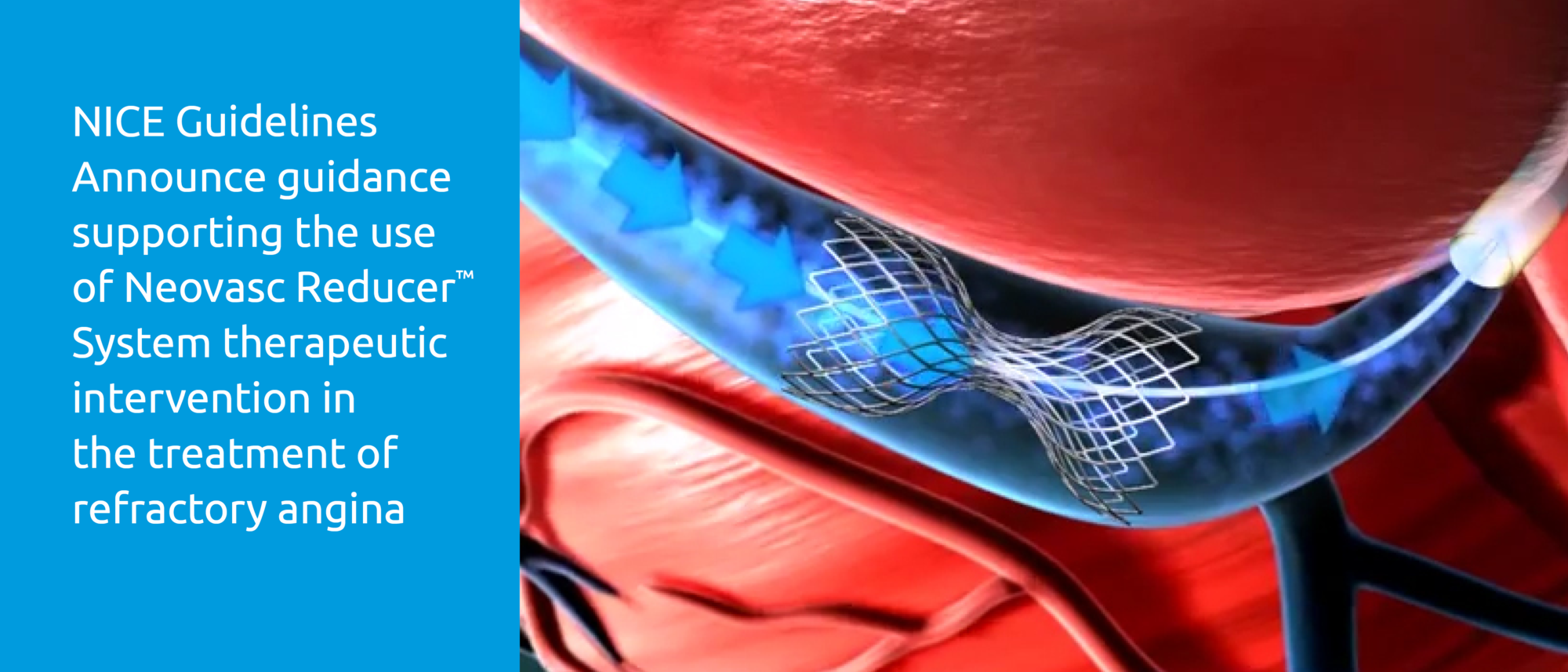
About Reducer
- The Reducer is CE-marked in the European Union for the treatment of refractory angina, a painful and debilitating condition that occurs when the coronary arteries deliver an inadequate supply of blood to the heart muscle, despite treatment with standard revascularization or cardiac drug therapies. It affects millions of patients worldwide, who typically lead severely restricted lives as a result of their disabling symptoms, and its incidence is growing.
- The Reducer provides relief of angina symptoms by altering blood flow within the myocardium of the heart and increasing the perfusion of oxygenated blood to ischemic areas of the heart muscle. Placement of the Reducer is performed using a minimally invasive transvenous procedure and is completed in approximately 20 minutes.
- While the Reducer is not approved for commercial use in the United States, the FDA granted Breakthrough Device designation to the Reducer in October 2018. This designation is granted by the FDA in order to expedite the development and review of a device that demonstrates compelling potential to provide a more effective treatment or diagnosis of life-threatening or irreversibly debilitating diseases. In addition, there must be no FDA approved treatments presently available, or the technology must offer significant advantages over existing approved alternatives.
- Refractory angina, resulting in continued symptoms despite maximal medical therapy and without revascularization options, is estimated to affect 600,000 to 1.8 million Americans, with 50,000 to 100,000 new cases per year.
About Neovasc Inc.
- Neovasc is a specialty medical device company that develops, manufactures, and markets products for the rapidly growing cardiovascular marketplace. Its products include Reducer, for the treatment of refractory angina, which is not currently commercially available in the United States and has been commercially available in Europe since 2015, and Tiara™ for the transcatheter treatment of mitral valve disease, which is currently under clinical investigation in the United States, Canada, Israel and Europe. For more information, visit: neovasc.com.